Spanish flu: the killer that still stalks us, 100 years on
The pandemic wiped out up to 100 million lives, but scientists still struggle to explain what caused it. The answers could ensure that it never strikes again
One hundred years ago this month, just as the first world war was drawing to a fitful close, an influenza virus unlike any before or since swept across the British Isles, felling soldiers and civilians alike. One of the first casualties was the British prime minister and war leader, David Lloyd George.
On 11 September 1918, Lloyd George, riding high on news of recent Allied successes, arrived in Manchester to be presented with the keys to the city. Female munitions workers and soldiers home on furlough cheered his passage from Piccadilly train station to Albert Square. But later that evening, he developed a sore throat and fever and collapsed.
He spent the next 10 days confined to a sickbed in Manchester town hall, too ill to move and with a respirator to aid his breathing. Newspapers, including the Manchester Guardian, underplayed the severity of his condition for fear of presenting the Germans with a propaganda coup. But, according to his valet, it had been “touch and go”.
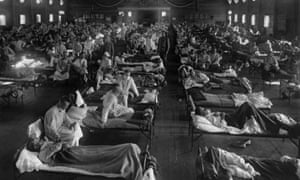
Lloyd George, then aged 55, survived, but others were not so lucky. In an era before antibiotics and vaccines, the “Spanish influenza” – so-called because neutral Spain was one of the few countries in 1918 where correspondents were free to report on the outbreak – claimed the lives of nearly 250,000 Britons. Cruelly for a nation that had seen the flower of British male youth mown down by German guns, the majority were adults aged 20 to 40. The mortality was the inverse of most flu seasons, when deaths fall most heavily on the elderly and the under-fives.
The global death toll was inconceivable: according to the most recent estimates, between 50 million and 100 million people worldwide perished in the three pandemic waves between the spring of 1918 and the winter of 1919. Adjusting for population growth, that is equivalent to between 200 million and 425 million today.
Unlike now, when reports of new bird flu outbreaks in south-east Asia are closely monitored by the World Health Organisation, there was no early warning system. Consequently, when it was reported in May 1918 that King Alfonso XIII was ill in Madrid, most people dismissed the Spanish flu as a joke. The main advice was to gargle with salt water and to isolate yourself until the fever had passed. However, these rules did not apply to munitions workers who were urged to “carry on” for the sake of the war effort.
As in other 20th-century epidemics and pandemics, such as HIV/Aids, Africans and Asians suffered proportionately more than Europeans and north Americans. Thus, while the average case mortality in the developed world was about 2%, in India, where 18.5 million perished, it was 6%, and in Egypt, where 138,000 died, it was 10%. In isolated regions with “virgin” populations with no immunity to flu, the impact was truly astonishing – in Western Samoa, for example, a quarter of the population was wiped out. By contrast, American Samoa recorded no casualties.
The severity of the pandemic and the peculiar death pattern puzzle scientists to this day. Few epidemiologists believe the pandemic began in Spain, pointing instead to pre-pandemic waves in Copenhagen and other northern European cities in the summer of 1918. Where the virus first leapt from birds to humans or some other mammal is even more perplexing, with some scientists favouring a Kansas point of origin and others northern France or China.
Earlier this year, in search of answers for a new podcast series, I travelled to Washington DC to interview one of the world’s leading experts on the 1918 pandemic. Jeffrey Taubenberger, a molecular pathologist at the National Institute of Allergy and Infectious Diseases, has been studying the Spanish flu virus for more than 30 years. In the late 1990s he succeeded in retrieving fragments of viral RNA from stored pathology specimens taken from American soldiers who had died of flu at US army camps in 1918 and an Inuit woman who been buried on a beach in Alaska, where the permafrost had preserved her lung tissue from decay.
Using modern molecular techniques, Taubenberger and his colleague, Anne Reid, amplified the fragments and, in 2005, published the virus’s genetic sequence. Their findings were a shock. Previously, epidemiologists had observed that flu pandemics were preceded or followed by outbreaks of influenza-like illnesses in dogs, cats, and horses. It was also known that from time to time flu viruses could infect pigs and, of course, humans, and that wild flu viruses circulated in migratory waterfowl. However, when Taubenberger analysed the genome of the Spanish flu, he found that most of its genes were derived from a bird flu virus. Indeed, Taubenberger considered the H1N1 virus so “avian-like’” he could not discount the possibility that it had transmitted directly from birds to humans shortly before 1918 – and perhaps as early as 1916.
Taubenberger’s discovery raised the terrifying possibility that, in the future, some other avian influenza virus – like the H5N1 bird flu then circulating in south-east Asia or the H7N9 flu currently causing sporadic human infections in China – might suddenly acquire the ability to trigger a similarly devastating pandemic. It also begged the question, why bring the Spanish flu back to life, and what if the virus escaped the laboratory and fell into the hands of terrorists?
To prevent that happening, Taubenberger and other scientists with access to the freezer containing the virus are screened by the FBI and must wear double-gloves, a respirator and a full body suit – like the ones worn by medical workers during the west African Ebola epidemic. They must also submit to an iris scan. “It’s really the equivalent to top secret clearance,” he says.
Continued experimentation is necessary for the development of vaccines and other medical interventions. In mice, the H1N1 Spanish flu is extremely virulent, generating 39,000 times more virus particles than a modern flu strain. By targeting the inflammatory response, Taubenberger has shown that mice can be protected. But scientists are a long way from finding a cure for flu, much less a universal vaccine against seasonal and future pandemic strains.
Frustratingly, it is still not known where and when the Spanish flu acquired its avian genes and first began spreading in humans. The genes map most closely to wild waterfowl from north America but, despite examining the Smithsonian Institute’s extensive bird collections, Taubenberger was unable to find viable autopsy remains from before 1918.
One theory is that the so-called “spillover” event occurred in early 1918, not far from an army camp in Kansas that supplied soldiers to the American Expeditionary Force. Certainly, there were explosive outbreaks of an influenza-like illness at Camp Funston, Fort Riley, in March 1918, followed by similar outbreaks along the eastern seaboard of the US and on the transatlantic troop carriers that ferried American troops to France. However, the earliest fragments of the pandemic virus obtained by Taubenberger date from May 1918, so there is no way of telling whether outbreaks prior to this were caused by the pandemic strain, as opposed to an ordinary seasonal influenza.
A rival theory, favoured by the British virologist John Oxford, is that the pandemic began at Étaples, a huge British military camp an hour south-west of Boulogne. With accommodation for up to 100,000 soldiers, Étaples lay on a migratory bird flyway close to the Somme estuary and had all the necessary conditions for a spillover event: wild waterfowl, plus chickens and pigs, living in close proximity to men packed into airless barracks. Étaples also had several hospitals where soldiers whose lungs had been compromised by mutagenic gases deployed on the battlefield were evacuated for treatment.
In the winter of 1917, several hundred British soldiers collapsed with influenza-like symptoms and medics at Étaples recorded 156 deaths. At the time, the epidemic was labelled “purulent bronchitis” because of the yellow pus that oozed from the larger airways of the lungs at autopsy (some medics thought it resembled the lung damage from phosgene gas).
Another prominent feature was cyanosis, a distinctive purple-blue discolouration of the lips, ears and cheeks, caused by the loss of oxygen to the heart. Cyanosis was also a hallmark of the pneumonias associated with the Spanish flu – an observation that persuaded doctors writing in the Lancet in 1919 that it and purulent bronchitis had been “fundamentally the same condition”.
Another puzzle is why, in some cases of pneumonia associated with the Spanish flu, the onset was rapid and the lung damage highly localised, while in others, the infection resembled an aggressive bronchopneumonia with extensive haemorrhaging and swelling throughout the lungs. These features have never been adequately explained, with some pathologists arguing that the virus triggered an unusual auto-immune response known as a cytokine storm and others that extensive lung damage is better explained by bacterial infections that followed the flu – a big danger in the days before antibiotics.
But perhaps the biggest unanswered question is why the Spanish flu proved so deadly to young adults. Here, present-day science has hypotheses but no good answers. One suggestion is that the elderly enjoyed greater immunity because, as children, they had been exposed to a pandemic virus with a similar genetic makeup to the H1N1 Spanish flu. Conversely, those aged 28 and over had an immunological blind spot because their first exposure had been to the 1890 “Russian flu”, an H3 virus with a completely different configuration of genes. Or it could be that the unusual mortality pattern seen in 1918 was the result of an as yet unidentified environmental exposure or stressor peculiar to young adults at the time.
Answering those questions is important because genes from the Spanish flu continue to circulate in human and pig populations to this day. Some of these genes are direct descendants of the 1918 virus; others have reassorted with other pandemic viruses, such as the 1968 Hong Kong flu and the hybrid H1N1 virus responsible for the 2009 swine flu pandemic. As Taubenberger puts it: “[The outbreak of] 1918 set up a very successful introduction of a bird-like virus in humans that has never gone away in 100 years. It really was the mother of all pandemics.’
Mark Honigsbaum is the author of Living With Enza: The Forgotten Story of Britain and the Great Flu Pandemic of 1918. His podcast, Going Viral: The Mother of all Pandemics, is available at Libsyn and other podcast providers. @GoingViral_pod.
The pandemic was especially hard on children, perhaps more than any other segment of the population. Take Ada Darwin, who was seven when the “Spanish Lady” called at the house in Manchester’s Moss Side she shared with her mother and five siblings.
“It was Sunday 17 November that I was put to bed,” Darwin recalled when I interviewed her at her home in Chester in 2005. “I remember this great big headache and telling my mother to ‘stop my sister Norah chattering, it’s making my head hurt’.”
The next to fall ill was Ada’s mother, Jane Berry, and her baby sister, Edith, followed by her younger brothers, Austin, two, and Noel, four. With the whole family stricken, Ada’s grandmother was summoned to their home. But by the time she arrived, Ada’s mother was covered in dark blue patches – an indication she had cyanosis – and the prognosis was hopeless. She died the next day – Wednesday 20 November – followed, three days later, by Noel. Jane Berry was just 34.
Then, on 25 November, Darwin learnt that her 38-year-old father, Frederick Berry, a member of the Royal Army Medical Corps, had also died, most likely after catching the flu at Salford military hospital, where he had stayed on after the Armistice to nurse wounded soldiers. He was buried with full military honours at Manchester Southern Cemetery on 29 November, along with Darwin’s mother and Noel. Aged 94, Darwin could still recall the triple funeral cortège as it passed her primary school.
“It’s like a film in my head,” she told me in 2005. “There were the black horses with the plumes made from ostrich feathers, then the gun carriage with my dad’s coffin covered with the union flag. My mother’s coffin was in a big glass hearse with Noel’s coffin under the driver’s seat. My grandma told us my mother had gone to Jesus, but I said, ‘Jesus has got plenty of people, I want my mummy’.”
Darwin was not the only child to be orphaned by the flu. In Cape Town, observed one eyewitness, the autumn wave “made orphans of between two to three thousand children”.
In London, meanwhile, it is estimated that 16,000 people perished between September and December 1918, the majority of them young men and women. The result was that 1919 would be the first year since records began that Britain’s death rate exceeded its birthrate.
Today, there are few people still alive to recall those dark days in November when, according to Manchester’s chief medical officer, James Niven, “it seemed as if it would not be possible to get coffins for the dead, or gravediggers to dig the graves”. All the more reason why, in the centenary year of the pandemic, it is worth recalling the experiences of Darwin and other survivors of the Spanish Lady.




No comments:
Post a Comment